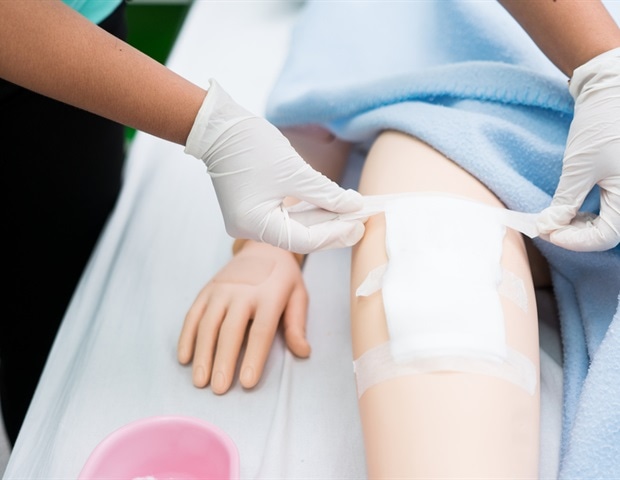
SOLASCURE Ltd (SolasCure), a biotechnology firm growing a novel therapy to remodel power wound therapeutic, right this moment introduced the initiation of a brand new Section II scientific trial, CLEANVLU2. The enrolment of the trial’s first affected person marks a brand new scientific milestone within the improvement of its investigational product, Aurase Wound Gel, for the therapy of power wounds.
Aurase Wound Gel is a hydrogel releasing Tarumase, a recombinant enzyme initially cloned from medical maggots that selectively targets fibrin, collagen and elastin in wounds to advertise therapeutic by means of debridement and wound mattress preparation. CLEANVLU2 provides additional perception to the profitable Section IIa scientific research, CLEANVLU, which established proof-of-concept, a robust security profile and pain-free utility. The brand new randomized managed trial will discover the efficacy of Tarumase at larger focus, in sufferers with venous leg ulcers.
Persistent wounds have an effect on round 100 million individuals globally, representing a big healthcare problem with restricted protected, pain-free, and efficient remedies. Scientific information counsel that, after 6 weeks of the present commonplace of care therapy, total wound closure is achieved in as little as 41% of power or hard-to-heal wounds. This trial will generate key efficacy information to additional show Aurase Wound Gel’s potential as the primary therapy to deal with all facets of wound mattress preparation together with debridement, informing buyers and potential strategic companions.
The trial is being run in affiliation with South Leicestershire Medical Group, UK, as a part of its neighborhood service. As soon as the CLEANVLU2 research is accomplished, an additional Section II research is deliberate over an extended interval, with stratification for elements which will have an effect on debridement and wound therapeutic, earlier than shifting into confirmatory scientific Section III trials for regulatory approval.
This trial is pivotal for absolutely demonstrating proof-of-concept of efficacy for Aurase Wound Gel with a stronger focus of the enzyme Tarumase. This may assist us to determine that the product can obtain full debridement in 6-9 purposes aligned with commonplace of care and have a constructive affect on the speed of therapeutic. That is what the market has been ready for and brings Aurase Wound Gel a step nearer to offering reduction to these affected by power wounds worldwide.”
Andy Weymann MD, MBA, Chairman of the Board, SolasCure
